We also rewrite the second (we do not make too serious an error if we accept only integral numbers of revolution):
1 second ≈ number of the revolution periods * revolution period

XXX
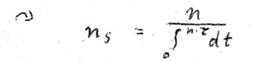
Now, since we do not want to sum up many electrons, we will use another concept that enables us to express ns for one electron. For this purpose we provide the following example: If each of 6 electrons performs one revolution per second, the following equation will apply:
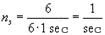 .
.
The result will be the same if one electron is made to orbit six times faster:
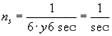
This is what we have achieved: we consider the number n as a number of revolutions that can be performed by one electron in a defined period and now require this number to be an integer. We justify this requirement by our magnetic field theorem that forbids the discussion about the existence of a magnetic field at all unless at least one complete revolution has been performed. Thus, we have illustratively introduced the main quantum number n.
Thus, we write:
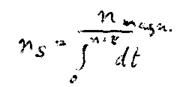
Consequently, this equation should apply also for magnetic fields.